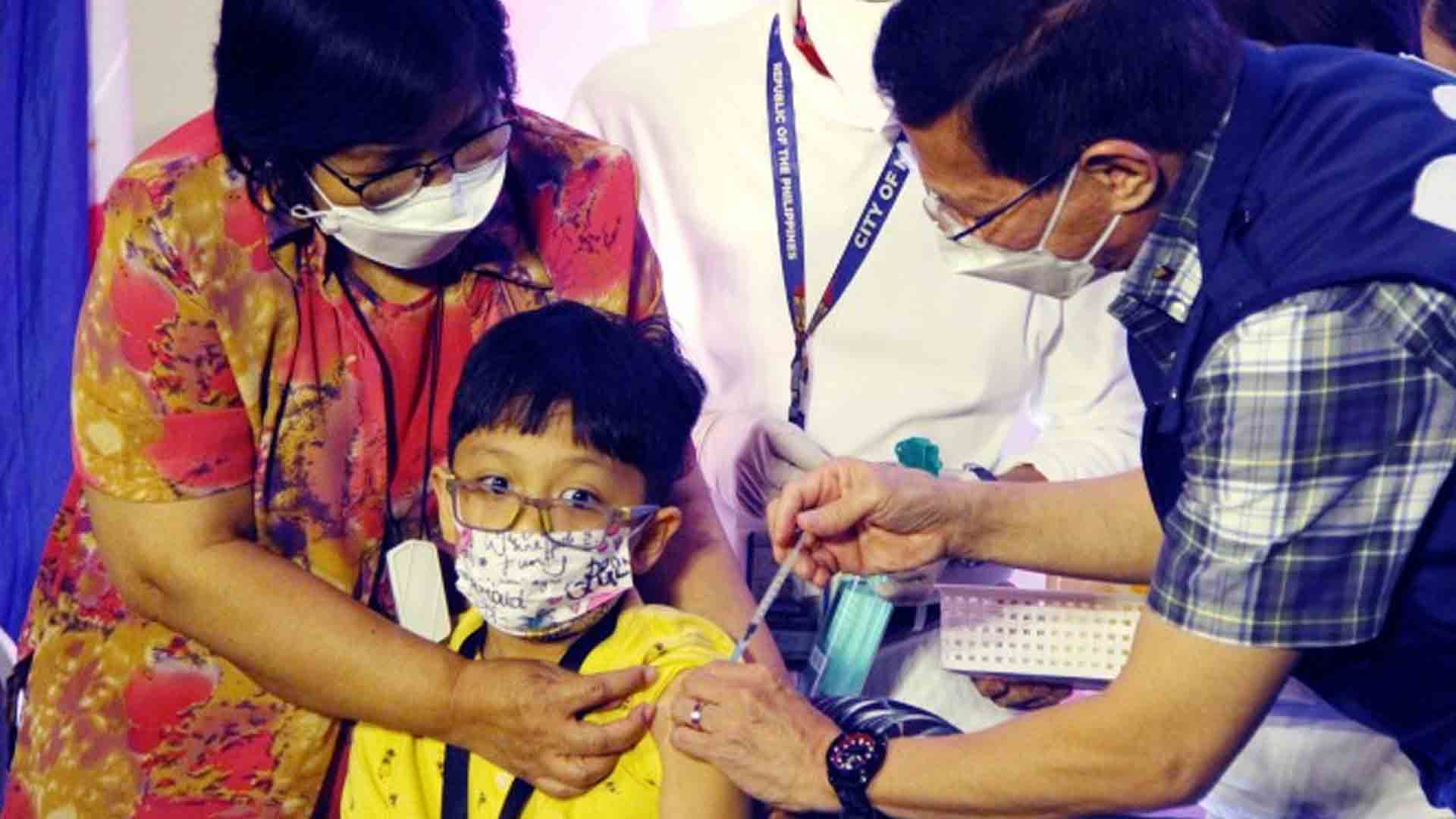
The vaccine expert panel (VEP) said Wednesday it is waiting for Covid-19 vaccine manufacturers to apply with the Food and Drug Administration (FDA) an emergency use authorization (EUA) allowing their jabs to be used as booster shots for young children.
This, after the United States FDA has authorized the use of Pfizer Covid-19 booster dose for children ages 5 to 11 years old.
“Actually, hihintayin na mag-apply sila sa FDA, kung ‘di sila mag-a-apply wala tayong aaksyunan (we’ll wait for them to apply to FDA, there’s nothing for us to work on if they won’t apply),” VEP chairperson Dr. Nina Gloriani said in a televised public briefing.
The Covid-19 vaccination of children ages 5 to 11 years old started on February 7.
As of March 15, a total of 1,233,017 of the age group have been inoculated and 381,433 of them are fully vaccinated.
Earlier, National Vaccinations Operations Center chief Myrna Cabotaje said the Department of Health (DOH) is coordinating with the Department of Education and local government units to facilitate school-based Covid-19 vaccination in both public and private schools.
The National Task Force Against Covid-19 (NTF Against Covid-19), in a resolution dated May 12, urged public and private schools for basic education to carry out Covid-19 vaccination.
The inoculation activities should be conducted “with the informed consent given and signed by the parent or guardian, and the assent given by the learner pursuant to applicable DOH guidelines,” it said. (PNA)